Abstract
Background: Immune checkpoint inhibitors have revolutionized cancer therapeutics. These drugs block the interaction between PD -1/PDL-1, thus, promoting T-lymphocyte immune destruction of cancer cells. Checkpoint inhibitors are efficacious in cancer treatment and are generally well-tolerated in patients. However, activation of the immune system reacts with both tumor and healthy cells leading to toxicities known as immune-related adverse events(irAEs). Pulmonary irAEs, especially pneumonitis, are rare and difficult to diagnose. Previous studies have shown a 4% (single agent), and 10% (combination studies) risk of developing pneumonitis in solid tumors with exposure to checkpoint inhibitors (Sun et al., 2019, Naidoo et al., 2017). Although rare, pneumonitis has been reported as the most frequent fatal event, accounting for 35% of fatal toxicities from anti-PD-1/PD-L1 (Wang et al., 2018). However, the risk of developing pneumonitis when used for hematologic malignancies is not known especially in classical Hodgkin's Lymphoma (cHL) where it is used both in monotherapy and in combination with chemotherapy in the upfront and relapsed setting, often in patients who subsequently receive radiation and/or autologous stem cell transplant.
Objective This study aims to estimate the risk of developing pneumonitis with exposure to immune checkpoint inhibitor (ICI) monotherapies to treat hematologic malignancies: nivolumab and pembrolizumab (anti-PD-1 drugs), durvalumab, and atezolizumab (anti-PD-L1 drugs).
Methods We retrospectively reviewed the Food and Drug Administration Adverse Events Reporting System (FAERS), a pharmacovigilance (PV) database from 2012 to 2021. Statistical analysis involved a disproportionality analysis using a reported odds ratio (ROR). We use the ROR to assess the risk of pneumonitis with exposure to the five ICIs. OpenVigil, a software package used to analyze pharmacovigilance data, enabled the calculation of the ROR. The study reports a confidence interval of 95% to evaluate the precision of the ROR; ROR greater than one, p<0.05, and CI not including one indicated a greater odd of developing pneumonitis with an ICI of interest compared with other drugs in the database.
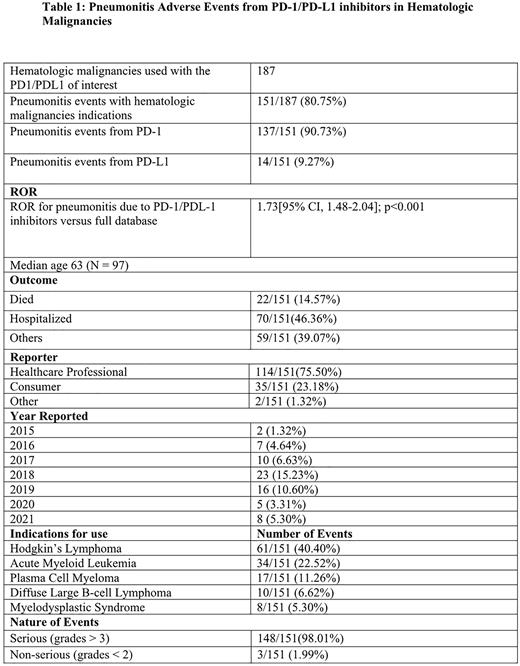
Results The total number of events reported in FAERS between 2012 and 2021 was 17,273,403. Of these, 88,099(0.51%) were attributed to the PD-1inhibitors (nivolumab and pembrolizumab) and 21,905(0.13%) to PD-L1 inhibitors of interest. FAERS reported about 187 patients with hematologic malignancies treated with the five ICI monotherapies. Of these, 151(80.75%) patients developed pneumonitis. Ninety percent of the pneumonitis events were due to anti-PD-1drugs; (33(21.85%) with pembrolizumab and 103 (68.21%) with nivolumab). In contrast, 9.27% of the events were from the anti-PD-L1 drugs (7 (4.64%) with both durvalumab and atezolizumab). The combined risk of developing pneumonitis was significant at 1.73[95% CI, 1.48-2.04] p<0.001. About 148(98.01%) of the events were serious (grades > 3), and three (1.99%) were non-serious (grades <2). Healthcare professionals report about 80% of the events in FAERS; this may lead to a biased overreporting of higher-grade adverse events. Forty-six percent of the patients were hospitalized from the pneumonitis event, while 14.56% died from the event. Hodgkin's lymphoma was the most common indication for using the ICIs, 61 (40.40%). The most common regions to report these events were the United States, 110(72.85%), and Japan, 6(3.97%).
Conclusion This retrospective study shows a risk of developing pneumonitis with exposure to checkpoint inhibitors. The risk is more significant for PD-1 inhibitors than for PD-L1 inhibitors. Patients with Hodgkin lymphoma are most affected, and while rare, this is a potentially fatal complication of therapy with ICIs. Thus, early detection is essential to initiate management, thus, minimizing patient morbidity and mortality from the event. Given the limitations of the PV database, this data will be compared with actual world data (RWD) at the upcoming meeting in December.
Disclosures
Ahmed:Myeloid Therapeutics: Consultancy; Chimagen: Consultancy, Research Funding; Xencor: Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees; Tessa Therapeutics: Consultancy, Research Funding; Seagen: Research Funding; Merck: Research Funding. Lee:Korean Society of Cardiology: Honoraria; Deloitte: Honoraria; Guidepoint Global: Honoraria; Curio Science: Honoraria; Cancer Experts: Honoraria; Olson Research: Honoraria; Octernal Therapeutics: Research Funding; Pharmcyclics: Research Funding; Celgene: Research Funding; Janssen: Honoraria; Briston-Myers Squibb: Research Funding; Seagen: Research Funding; Takeda: Research Funding; Century Therapeutics: Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.